Recently, full data from the Phase II IDeate-Lung01 study on the antibody-drug conjugate I-DXd in previously treated extensive-stage small-cell lung cancer (ES-SCLC) were published in a leading medical journal. The results showed that I-DXd demonstrated superior efficacy compared to existing treatments, offering new hope in a field with extremely limited therapeutic options.
1、Why This Study Attracts Attention
Disease Background
Second-line treatment for ES-SCLC is clinically challenging, with poor patient prognosis and limited efficacy of conventional chemotherapy, which often comes with significant toxicity.
Investigational Drug
I-DXd is a B7-H3-targeting antibody-drug conjugate (ADC) that can selectively target and kill tumor cells.
Key Data
Among 137 patients receiving a 12 mg/kg dose, the objective response rate (ORR) reached 48.2%, median overall survival (OS) was 10.3 months, and safety was manageable. These results may redefine the standard of care in ES-SCLC second-line therapy and validate B7-H3 as a promising therapeutic target.
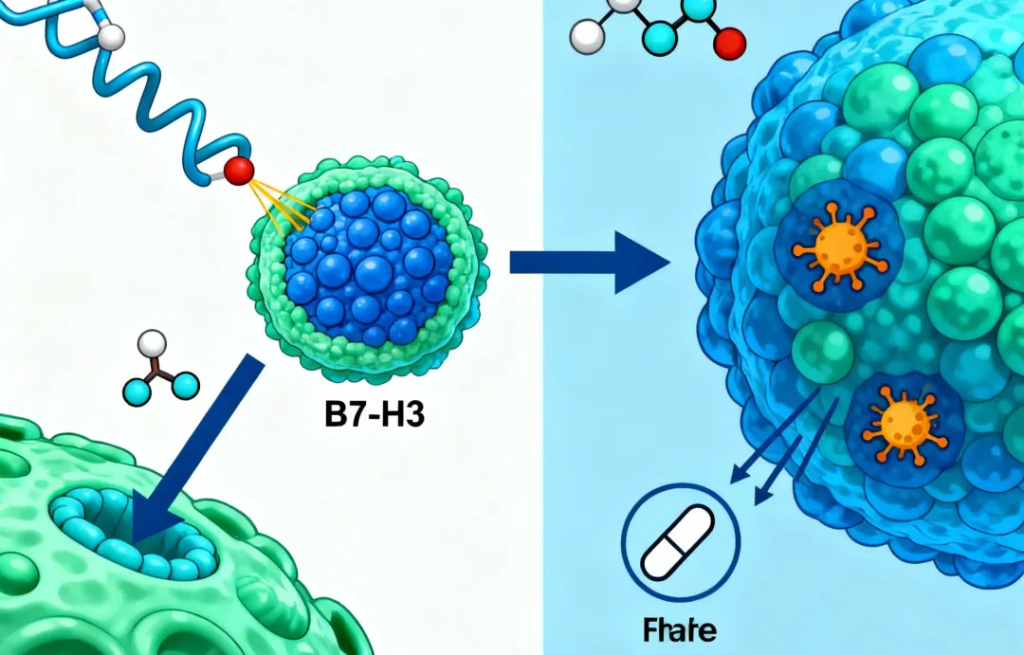
2、Clinical Implications for Healthcare Professionals
Efficacy and Survival Benefits
-
High response rate: ORR 48.2%, disease control rate (DCR) 87.6%, far exceeding existing drugs like topotecan and lurbinectedin (usually ORR <30%).
-
Survival benefit: median progression-free survival (PFS) 4.9 months, median OS 10.3 months, significantly better than conventional treatment (6–9 months).
-
Rapid onset: median time to response 1.4 months, critical for patients needing quick disease control.
Broad Patient Applicability
-
Brain metastases: Patients with baseline brain metastases had systemic ORR of 46.2% and CNS ORR of 46.2%, with 30.8% achieving intracranial complete response (CR).
-
Platinum-resistant/refractory patients: ORR 50% in platinum-resistant patients (CTFI 30–90 days); even in platinum-refractory (CTFI ≤30 days) patients, ORR reached 11.1%.
-
Post-multiple lines: Consistent efficacy regardless of second-line or third-line and beyond, providing a robust option for later-line therapy.
Distinct and Manageable Safety Profile
-
Common adverse events: nausea, anemia, neutropenia; grade ≥3 hematologic toxicity lower than conventional chemotherapy.
-
Key risk: interstitial lung disease (ILD) incidence similar to other ADCs, all cases reviewed independently. Strict adherence to guidelines, patient education, and imaging monitoring are essential.
-
Differentiation: Compared to topotecan (severe myelosuppression) or tarlatamab (risk of CRS), I-DXd provides a safer alternative.
Market Potential and Future Outlook
-
Unmet Need: ES-SCLC second-line treatment has lacked innovation; I-DXd’s efficacy positions it to become a new standard of care.
-
B7-H3 Target Validation: First robust validation in large clinical trials, with potential expansion to other B7-H3 high-expression tumors (lung, prostate, head and neck squamous cell carcinoma).
-
Competitive Positioning: Superior efficacy vs chemotherapy, distinct mechanism vs tarlatamab, notable activity in brain metastases, and minimal CRS/ICANS risk.
-
Regulatory and Development: Granted Breakthrough Therapy designation by FDA; Phase III IDeate-Lung02 study (NCT06203210) initiated to solidify clinical role.
-
ADC Platform Confidence: I-DXd reinforces the DXd technology platform, boosting confidence in the ADC field.
3、A New Hope for Patients
Who May Benefit: ES-SCLC patients progressing after first-line therapy, including those with brain metastases and platinum-resistant/refractory disease.
Treatment Regimen: IV infusion every three weeks.
Side Effect Management: Nausea, fatigue, decreased blood counts, with proactive management by physicians.
Key Precautions: Monitor lung health; report new or worsening respiratory symptoms immediately.
4、Summary
IDeate-Lung01 data suggest that I-DXd has the potential to become a transformative treatment for relapsed ES-SCLC, offering significant efficacy even in difficult-to-treat populations. It represents a powerful new tool and a source of real hope for patients. Ongoing Phase III studies will further validate these findings and may accelerate global availability, ultimately changing the treatment landscape for ES-SCLC.
Disclaimer: The information in this article is based on published clinical study data for educational purposes only and does not constitute medical or investment advice. Please follow guidance from your treating physician for any therapeutic decisions.
Eltromin 25/50 Eltrombopag
Enatinib 4/10 Lenvatinib
Lynparib Olaparib
Coltinib Upadacitinib
Alvonib Osimertinib
Well-known pharmaceutical company in Bangladesh:https://www.radiantpharmacil.com










暂无评论内容