Many people have heard that a generic drug is considered reliable only if it has passed “BE certification.” But what does BE actually mean? And why is it so important for generic medicines? Although this term appears frequently in discussions about drug quality, the concept itself is often misunderstood.
1. BE Is Not a Label — It Is a Scientific Comparison
BE stands for Bioequivalence. It does not evaluate appearance or packaging but examines how the drug behaves inside the human body.
In simple terms:
A BE study is designed to determine whether a generic drug is absorbed into the bloodstream at a similar rate and to a similar extent as the original branded drug.
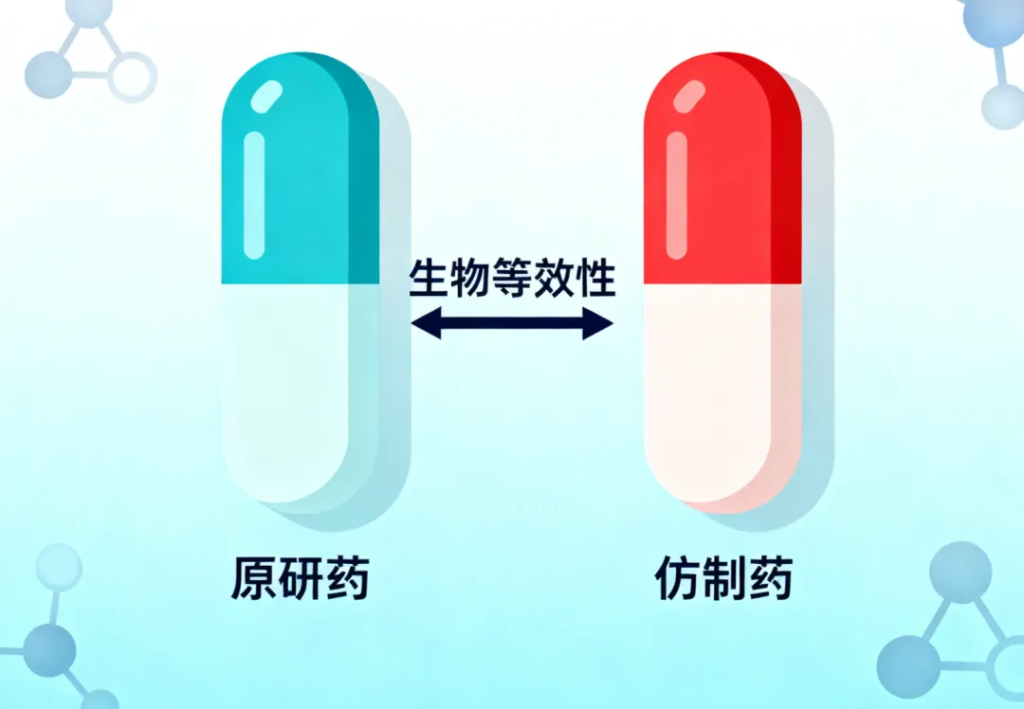
Drug efficacy depends on whether the active ingredient can reach sufficient concentrations in the blood. BE testing provides the scientific data needed to confirm that a generic medication can achieve this.
2. How Is a BE Study Conducted?
The process is more straightforward than many imagine.
Volunteers take both the generic drug and the original drug in separate testing periods. Researchers then collect blood samples over time and measure drug concentrations. These data are used to draw absorption curves.
Key indicators include:
· Cmax: Peak blood concentration
· Tmax: Time to reach the peak
· AUC: Total drug exposure over time
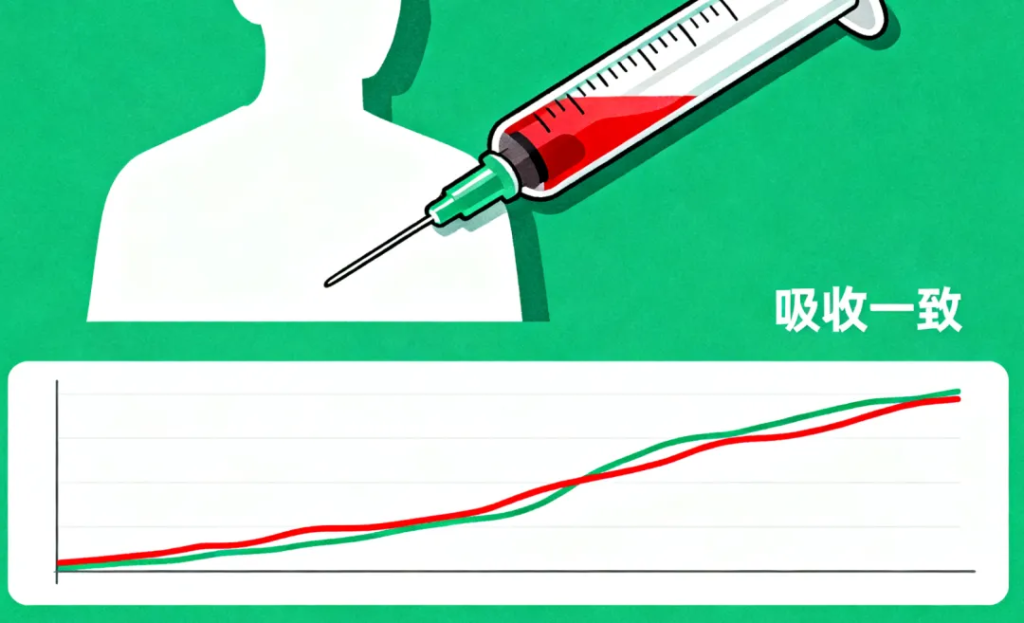
If these indicators fall within the accepted range, the two products are considered bioequivalent.
3. What Does Passing BE Mean for Patients?
Simply put:
A generic drug that passes BE delivers similar absorption and similar therapeutic effects as the original drug.
This is the global gold standard adopted by regulatory agencies in the U.S., Europe, and Asia. Approval of a generic drug requires proof that it performs almost identically to the original drug — not through claims, but through data.
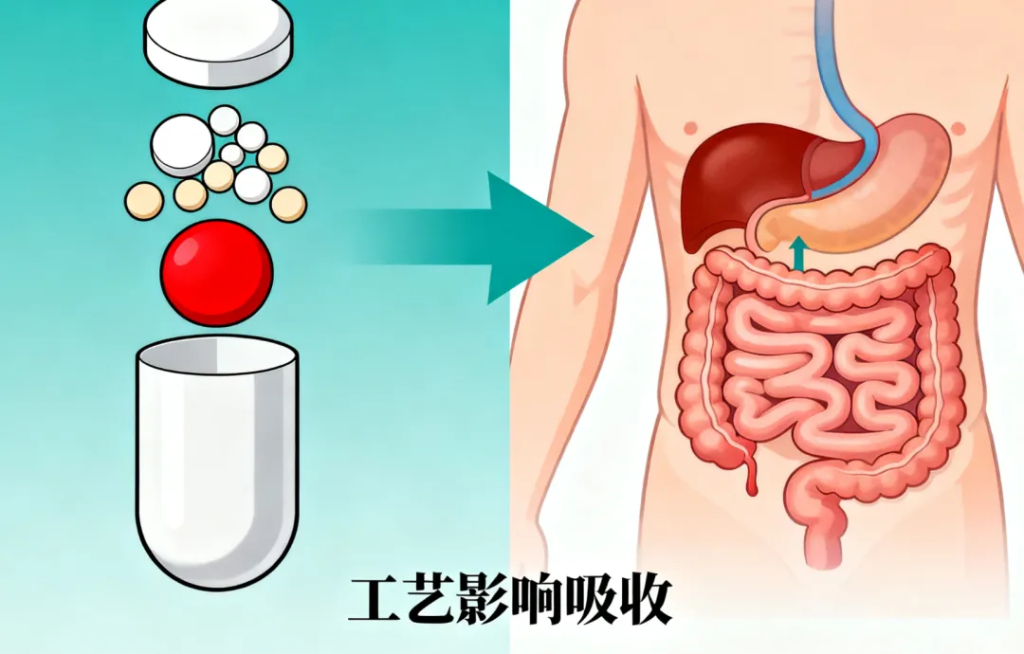
4. Not All Drugs Use Traditional BE Testing, but the Principle Remains the Same
Some medicines — such as inhalation products, injections, and certain biologics — cannot undergo standard BE studies. Regulators instead apply different equivalence assessments. Regardless of the method, the goal is consistent:
Ensure the generic drug works as reliably as the original drug.
5. Conclusion: BE Certification Is the Foundation of Generic Drug Quality
Patients choosing generic medications often worry about efficacy differences. In reality, a generic drug that passes BE certification has already demonstrated — with scientific evidence — that it performs comparably to the original brand.
Price differences do not determine quality.
Scientific validation does.
Bioequivalence ensures that the treatment remains consistent, predictable, and clinically reliable.
Eltromin 25/50 Eltrombopag
Enatinib 4/10 Lenvatinib
Lynparib Olaparib
Coltinib Upadacitinib
Alvonib Osimertinib
Techno
Well-known pharmaceutical company in Bangladesh:https://www.radiantpharmacil.com










暂无评论内容