Treatment of type 1 diabetes (T1D) is entering a transformative era. The first immunotherapy approved to delay clinical onset—Teplizumab—has received FDA approval in the United States. Other immune-based therapies also show promise in preserving β-cell function. Expanded public health screening using islet autoantibodies enables earlier diagnosis, reduces the risk of diabetic ketoacidosis, and allows patients to access disease-modifying therapies before requiring insulin.
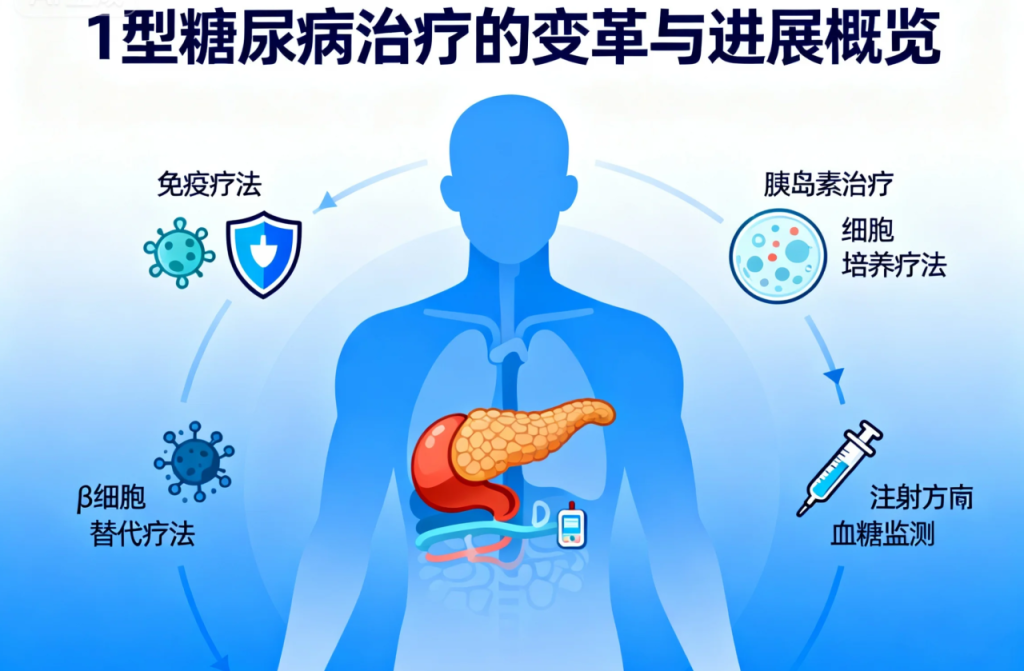
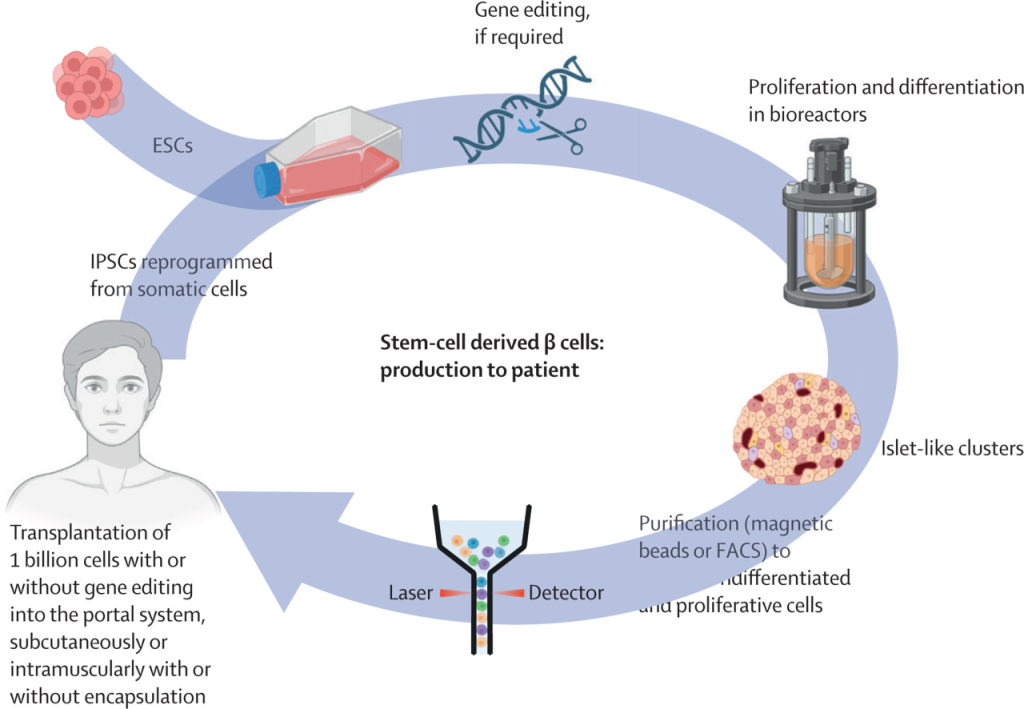
β-cell replacement therapy is evolving from traditional donor pancreas or islet transplants to stem cell-derived β-cell transplantation. Bioengineering approaches, such as cell encapsulation and gene editing, can create hypoimmunogenic cells, reducing the need for immunosuppressants—historically a major barrier. Patient-derived stem cells offer opportunities for personalized therapy. While these innovations currently benefit only a small number of patients, broad implementation remains a challenge.
Simultaneously, automated insulin delivery (AID) systems, integrating continuous glucose monitoring with insulin pumps, improve glycemic control. Next-generation insulins—including ultra-rapid, ultra-long-acting, and glucose-responsive formulations—minimize glucose fluctuations. These breakthroughs collectively provide new hope for long-term management and quality of life in T1D.
Introduction: A Century of Progress and a New Era
Since the discovery of insulin over a century ago, T1D treatment has dramatically advanced. Innovations include human insulin, insulin analogues, insulin pumps, AID (closed-loop) systems, and continuous glucose monitoring. Despite these improvements, individuals diagnosed before age 10 still have reduced life expectancy compared to later-onset T1D and non-diabetic individuals, and the risk of diabetes-related complications remains high.
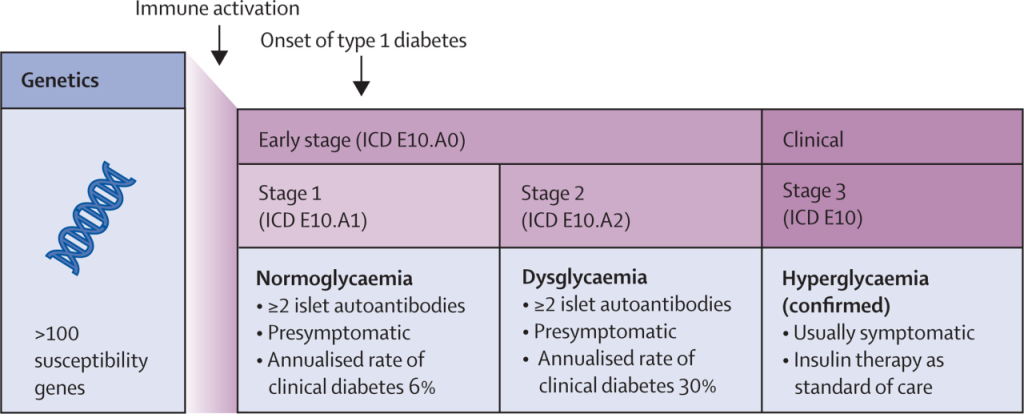
The discovery of islet cell autoantibodies in 1974 confirmed T1D as an autoimmune disease. Nearly 50 years later, FDA approval of the first disease-modifying therapy in the preclinical stage marks a new paradigm: treating T1D as an immune-mediated disease. Early intervention with immunotherapy aims to preserve β-cell function before the need for insulin.
Stem cell therapy has demonstrated proof-of-concept for replacing insulin-secreting cells, offering potential for a functional cure. Next-generation insulin therapies and AID systems enhance clinical efficacy, safety, and convenience, supporting individualized diabetes management.
Key Advances
1. Preventing or Delaying β-Cell Loss
T1D onset is characterized by progressive loss of β-cell function, primarily due to autoimmune destruction. Immunomodulatory therapy can delay or prevent clinical onset in asymptomatic individuals and protect residual β-cell function in newly diagnosed patients.
Screening and Early Detection:
-
High-risk populations (e.g., relatives of T1D patients) benefit from islet autoantibody screening.
-
Early-stage screening reduces diabetic ketoacidosis incidence and mitigates disease severity.
Disease-Modifying Therapy:
-
Teplizumab (anti-CD3 monoclonal antibody) delays T1D clinical onset by an average of 24 months.
-
Other immunotherapies, such as abatacept (CTLA-4-Ig), ATG, JAK inhibitors, anti-CD20, TNF inhibitors, and IL-12/23 inhibitors, show varying efficacy in preserving β-cell function post-diagnosis.
2. β-Cell Replacement Therapy
-
Stem cell-derived β-cell transplantation offers an alternative to donor organ transplantation.
-
Bioengineering techniques reduce immunogenicity and may limit the need for immunosuppressive therapy.
-
Patient-derived stem cells enable personalized treatment approaches.
3. Advanced Insulin Therapies and Automated Delivery
-
Ultra-rapid-acting insulin improves postprandial glucose control.
-
Ultra-long-acting insulin (weekly dosing) enhances adherence.
-
Glucose-responsive insulin supports physiological glucose regulation.
-
Automated insulin delivery systems improve glycemic targets and reduce patient burden.
Conclusion
T1D treatment is undergoing a paradigm shift: from insulin replacement alone to disease modification, β-cell replacement, and individualized glycemic management. These breakthroughs offer the potential to transform long-term outcomes and improve quality of life for patients.
Eltromin 25/50 Eltrombopag
Enatinib 4/10 Lenvatinib
Lynparib Olaparib
Coltinib Upadacitinib
Alvonib Osimertinib
Well-known pharmaceutical company in Bangladesh:https://www.radiantpharmacil.com
Article source:https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(25)01438-2/fulltext?dgcid=tlcom_carousel3_whod_reviews25_lancet










暂无评论内容