The U.S. Food and Drug Administration (FDA) has officially approved Novartis’ latest gene replacement therapy, Itvisma, marking a significant milestone in the treatment landscape for spinal muscular atrophy (SMA). This therapy is now authorized for patients aged two years and older with a confirmed mutation in the SMN1 gene.

Broader Treatment Access for SMA Patients
Prior to this approval, gene therapy options for SMA were limited by age. Novartis’ earlier treatment, Zolgensma, is currently approved only for pediatric patients under the age of two. Itvisma expands therapeutic access to a larger patient population, offering clinicians a new option for managing SMA in older children and certain adults.
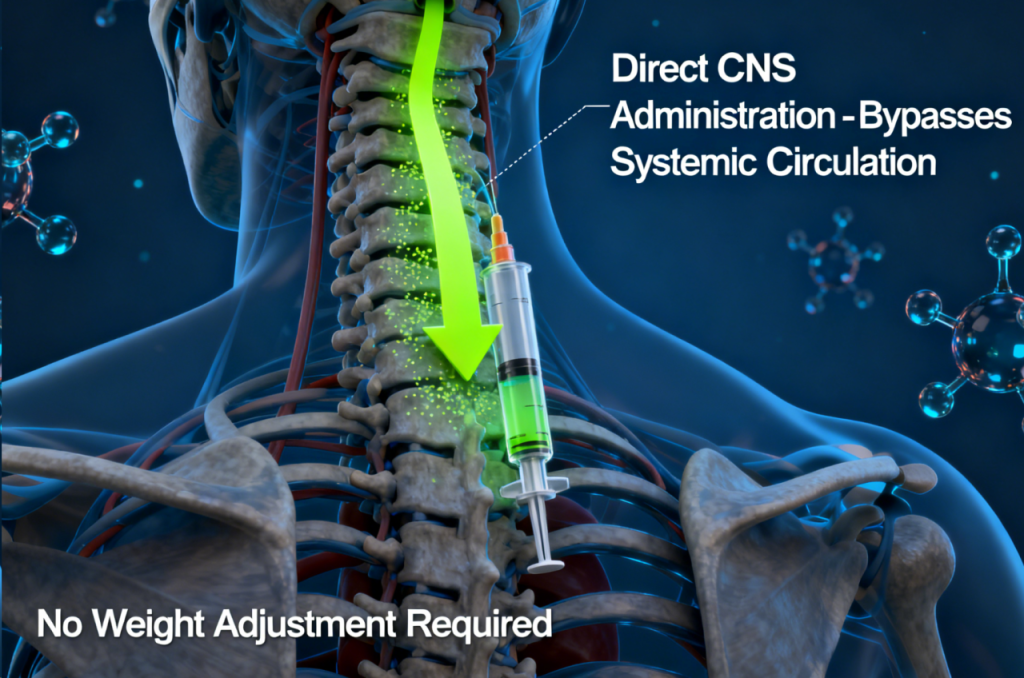
Refined Administration Method Targeting the Central Nervous System
Although Itvisma contains the same active ingredient used in Zolgensma, the delivery method is notably different:
-
Zolgensma: Administered intravenously, with dosage based on patient weight.
-
Itvisma: Delivered directly into the central nervous system through intrathecal administration and does not require weight-based dosing.
This targeted approach ensures the therapy reaches the spinal cord more directly, aligning with the pathology of SMA and potentially improving clinical consistency.
Evidence from Late-Stage Clinical Trials
In a pivotal late-stage clinical study, patients treated with Itvisma demonstrated a statistically significant improvement of 2.39 points on a standardized motor function scale. The results showed measurable enhancement in movement ability and a slowing of disease progression, underscoring the therapy’s clinical relevance.
Cost and Market Positioning
Novartis announced a wholesale acquisition cost of $2.59 million for Itvisma, higher than the $2.1 million price of Zolgensma. Despite the high cost, both therapies represent one-time gene replacement treatments designed to deliver long-term therapeutic benefit and potentially reduce the need for chronic medication.
Itvisma is now recognized as the first gene replacement therapy approved for the broader SMA population, reflecting the industry’s continued progress in rare disease innovation.
Understanding SMA and Its Impact
Spinal muscular atrophy is a rare genetic neuromuscular disorder caused by mutations in the SMN1 gene, leading to insufficient production of a protein essential for muscle function, breathing, swallowing, and basic movement. SMA remains the leading inherited cause of infant mortality, with approximately 9,000 individuals affected in the United States.
Both Itvisma and Zolgensma deliver functional SMN1 genes to replace the missing or defective gene, offering the potential for long-term disease modification.
Conclusion
The FDA approval of Itvisma represents an important advancement in SMA treatment. By expanding access to gene replacement therapy across a wider age range and offering a refined administration route, Itvisma provides new hope for patients and families confronting this challenging condition.
Eltromin 25/50 Eltrombopag
Enatinib 4/10 Lenvatinib
Lynparib Olaparib
Coltinib Upadacitinib
Alvonib Osimertinib
Techno
Well-known pharmaceutical company in Bangladesh:https://www.radiantpharmacil.com











暂无评论内容