The U.S. Food and Drug Administration has granted approval to Otsuka’s newly developed injectable therapy for patients diagnosed with primary immunoglobulin A nephropathy (IgAN), a progressive kidney disorder that may lead to renal failure if left unmanaged.
The drug, marketed under the name Voyxact, is a monoclonal antibody designed to reduce proteinuria and slow the progression of kidney damage. In a late-stage clinical study, the treatment achieved an average reduction of more than 50 percent in urinary protein levels over nine months, signaling a meaningful clinical impact for patients.
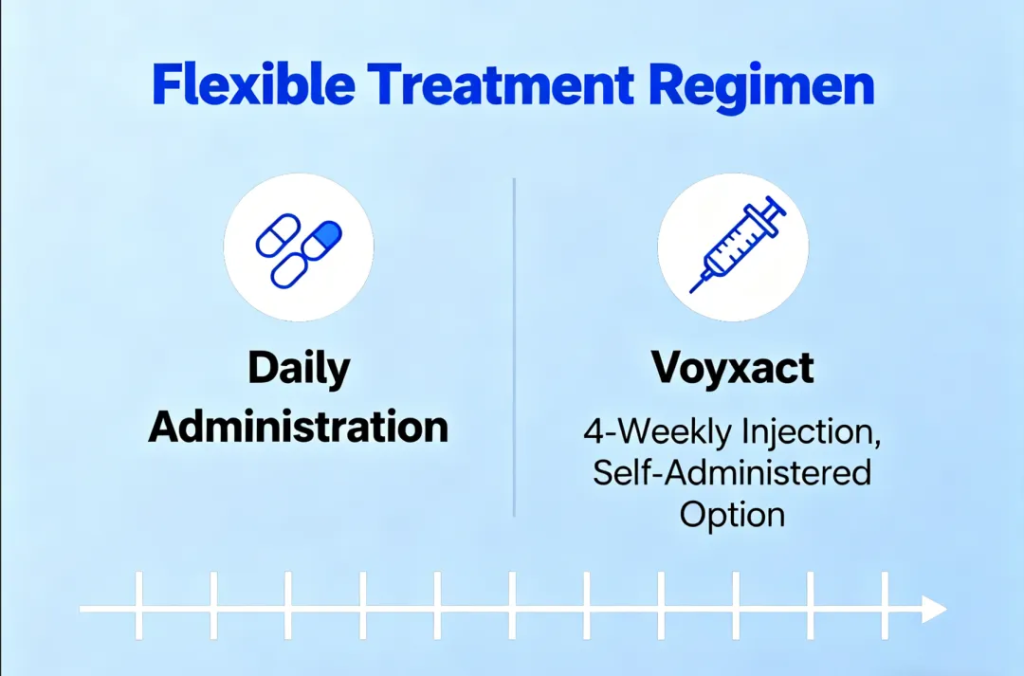
Voyxact offers an alternative to existing oral therapies. Unlike medications that require daily dosing, the newly approved injectable is administered once every four weeks and can be taken at home under a caregiver’s supervision. This provides added convenience for individuals requiring long-term disease management.
Current therapeutic options for IgAN primarily involve oral medications such as Novartis’ Fabhalta, Travere Therapeutics’ Filspari, and Calliditas Therapeutics’ Tarpeyo. With Voyxact entering the market, patients now have access to a treatment delivered through a different route and mechanism.
IgA nephropathy, also known as Berger’s disease, is characterized by the deposition of immunoglobulin A in the kidneys. This triggers inflammation, damages renal tissues, and results in blood and protein leaking into the urine. The condition often progresses slowly, making sustained medical therapy essential for protecting kidney function.
Otsuka is continuing a long-term clinical study to evaluate the therapy’s effect on kidney function over 24 months, with particular focus on changes in estimated glomerular filtration rate (eGFR). The study is expected to conclude in early 2026.
The approval of Voyxact is viewed as a significant addition to the IgAN treatment landscape, offering patients a new modality and expanding the tools available for clinicians managing this complex chronic condition.
Eltromin 25/50 Eltrombopag
Enatinib 4/10 Lenvatinib
Lynparib Olaparib
Coltinib Upadacitinib
Alvonib Osimertinib
Techno
Well-known pharmaceutical company in Bangladesh:https://www.radiantpharmacil.com










暂无评论内容