WHO Releases First GLP-1 Obesity Treatment Guideline, Signaling New Opportunities for the Global Pharma Industry
The World Health Organization (WHO) has issued its first global guideline for the use of GLP-1 receptor agonists in obesity treatment, marking a significant shift in the global understanding of obesity as a chronic and relapsing condition that requires long-term, structured medical care. The guideline comes at a time when more than one billion people worldwide are living with obesity, and obesity-related conditions led to an estimated 3.7 million deaths in 2024. Without effective interventions, global prevalence is expected to double by 2030.
GLP-1 Medicines Enter the Long-Term Obesity Care Framework
Following the inclusion of GLP-1 therapies in WHO’s Essential Medicines List (2025) for high-risk diabetes management, the new guideline provides conditional recommendations for their use in adult obesity care. According to WHO, GLP-1 medications may be prescribed to adults—excluding pregnant women—as part of a comprehensive obesity management plan that also includes behavioral therapy, healthy dietary practices, regular physical activity, and ongoing clinical support.

WHO Director-General Dr. Tedros Adhanom Ghebreyesus emphasized that GLP-1 therapies are not a standalone solution but can significantly assist individuals in managing obesity-related health risks. He noted that obesity requires lifelong care, and medication should be integrated into broader intervention strategies.
Implications for the Pharmaceutical Industry
The guideline is widely viewed as a catalyst for the next phase of growth in the global anti-obesity therapeutics market. GLP-1 innovations have already reshaped metabolic disease treatment, and WHO’s recognition further validates their clinical value.
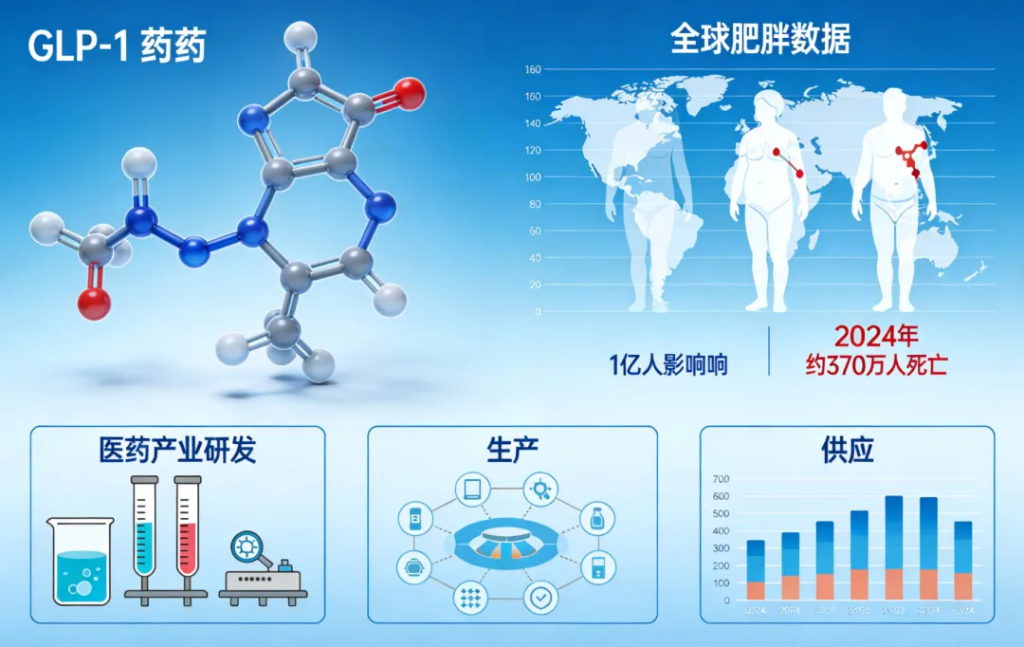
However, several challenges remain. Current global supply is insufficient to meet rapidly rising demand. Long-term safety data is still limited, and high treatment costs continue to restrict access in many regions. Projections suggest that fewer than 10 percent of eligible patients worldwide will have access to GLP-1 therapies by 2030 unless production capacity and affordability initiatives are significantly expanded.
Obesity Management Requires More Than Medication
WHO stresses that pharmacotherapy alone cannot solve the global obesity crisis. The guideline highlights a multi-level strategy consisting of:
-
Health-supportive environments: Improved access to nutritious foods, physical activity, and preventive services.
-
Early protection of high-risk groups: Screening, risk assessment, and targeted interventions.
-
Comprehensive, person-centered care: Lifelong medical, behavioral, and community support.
This framework underscores that sustainable obesity control depends on the combined efforts of policy, medical care, and lifestyle support.
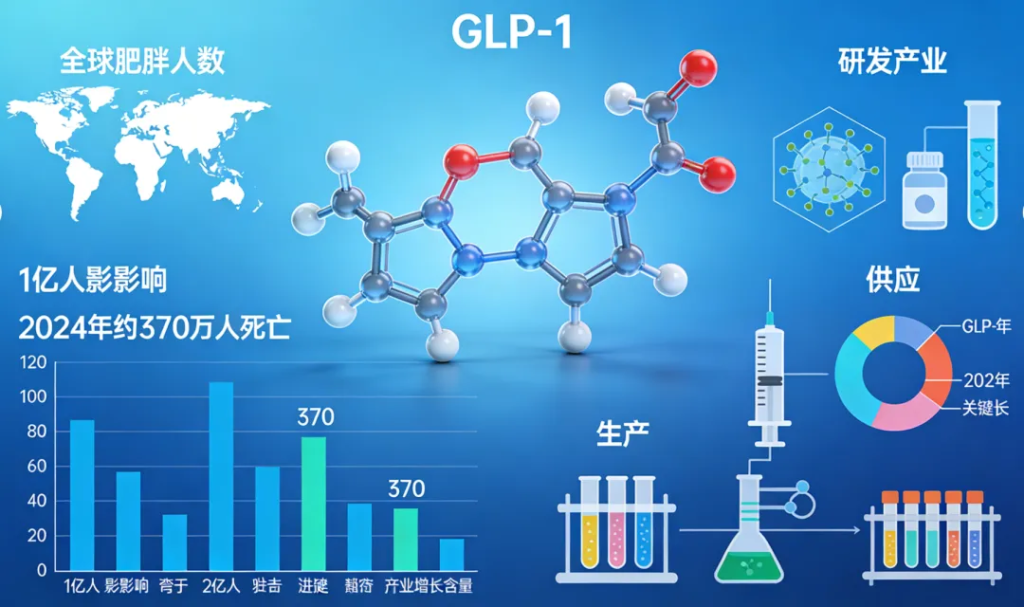
Advancing Access and System Preparedness
To ensure meaningful benefits from GLP-1 therapies, WHO calls on governments, regulatory bodies, manufacturers, and health systems to collaborate on:
-
Affordable pricing mechanisms, including pooled procurement and tiered pricing
-
Strengthening regulatory pathways
-
Scaling global manufacturing capacity
-
Training health professionals in long-term obesity management
WHO will work with Member States through 2026 to develop equitable prioritization frameworks that ensure patients with the greatest clinical need receive access first.
A Defining Moment for Pharma Innovation
The new guideline positions obesity care as a central frontier for the next generation of therapeutic innovation. As global health systems prepare for broader adoption of GLP-1 therapies, pharmaceutical companies are expected to play a pivotal role in expanding access, improving affordability, and scaling technologies that support effective obesity management at the population level.
Eltromin 25/50 Eltrombopag
Enatinib 4/10 Lenvatinib
Lynparib Olaparib
Coltinib Upadacitinib
Alvonib Osimertinib
Techno
Well-known pharmaceutical company in Bangladesh:https://www.radiantpharmacil.com










暂无评论内容