ProstACT Global Phase 3 Update: First Patient Dosed, Part 1 Preliminary Data Plans Confirmed
Telix recently announced that its lead prostate cancer candidate TLX591 (lutetium-177 rosopatamab tetraxetan) has dosed the first patient in Part 2 (randomized treatment expansion) of the ProstACT Global Phase 3 trial at the Australian Prostate Centre (APC) in Melbourne. This trial targets patients with metastatic castration-resistant prostate cancer (mCRPC), marking an important milestone for Telix’s late-stage prostate cancer therapeutics.
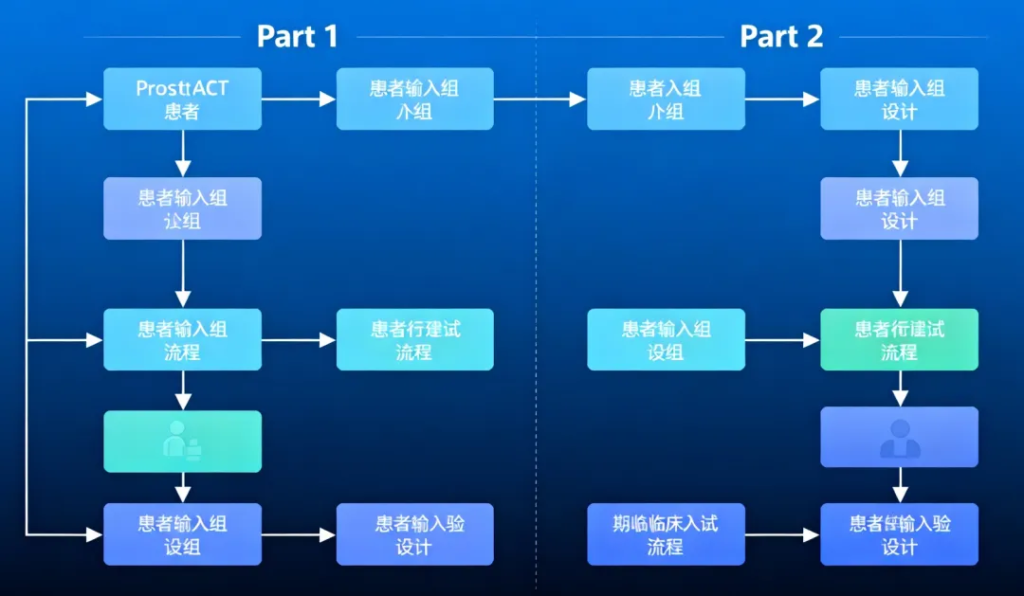
Highlights of ProstACT Global Trial
ProstACT Global is the first international, multicenter Phase 3 study combining a PSMA-targeted radio antibody-drug conjugate (rADC) with standard-of-care (SOC) therapy in a randomized design. The study includes:
-
Part 1: Safety and dosimetry lead-in, completed with 30 patients.
-
Part 2: 2:1 randomized global expansion, targeting approximately 490 patients, currently recruiting in Australia, New Zealand, and Canada.
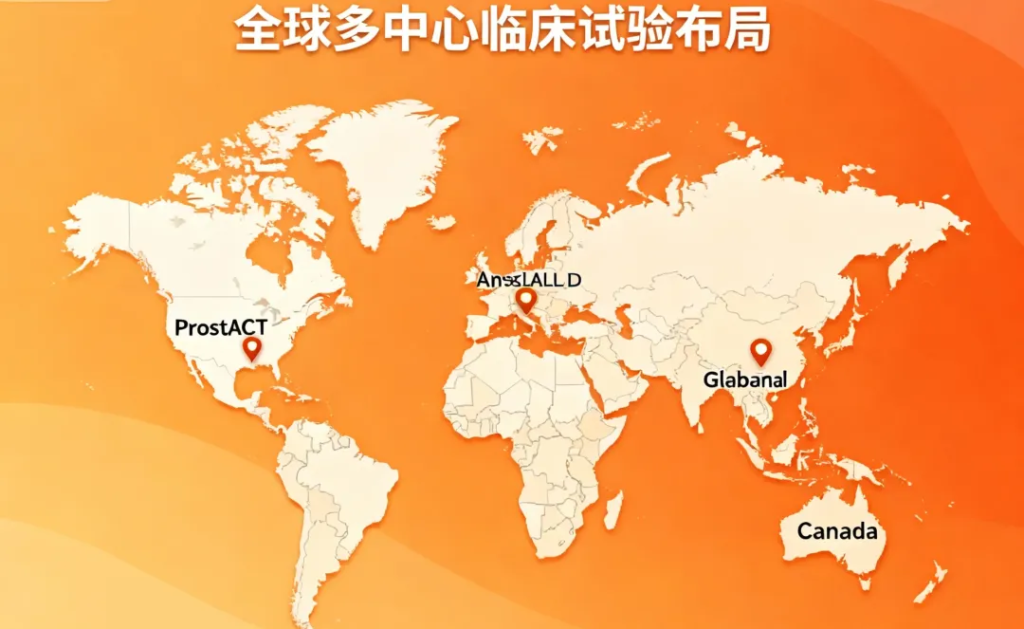
Telix plans to expand the trial to the U.S., Europe, China, Japan, Singapore, South Korea, Turkey, and the U.K. Preliminary Part 1 data will be submitted to the FDA and EMA to enable global expansion.
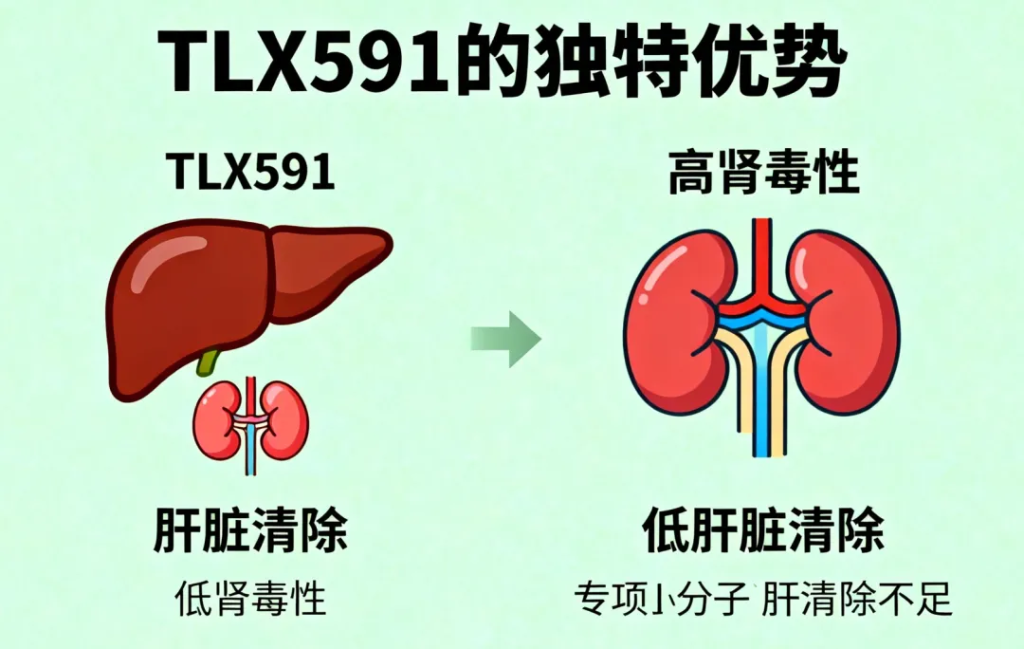
Innovative Advantages of TLX591
Compared to existing PSMA-targeted small molecule radioligand therapies (RLT), TLX591 shows unique pharmacology:
-
Liver clearance, minimal kidney toxicity – long-term follow-up shows no significant acute or delayed nephrotoxicity.
-
Low salivary and lacrimal gland uptake – reduces dry mouth and dry eye, common in current PSMA RLTs.
-
Combination with SOC – used with abiraterone, enzalutamide, or docetaxel, providing new options for mCRPC patients.
Clinical Outlook
Dr. David N. Cade, Telix Chief Medical Officer, commented: “Dosing the first patient into Part 2 of the randomized treatment expansion is a major milestone. We look forward to submitting Part 1 preliminary data to the FDA and EMA in the coming months.”
The global Phase 3 trial may offer mCRPC patients improved efficacy, safety, and tolerability, representing an important step forward in prostate cancer care.
Conclusion
The ProstACT Global Phase 3 trial marks a key development stage for PSMA-targeted rADC therapy in mCRPC. With trial expansion and upcoming Part 1 data disclosure, TLX591 has the potential to become a significant new option for advanced prostate cancer treatment.
Reference: ProstACT Global Study Details
Note: TLX591 has not received marketing authorization in any region.
Eltromin 25/50 Eltrombopag
Enatinib 4/10 Lenvatinib
Lynparib Olaparib
Coltinib Upadacitinib
Alvonib Osimertinib
Techno
Well-known pharmaceutical company in Bangladesh:https://www.radiantpharmacil.com
Article source:https://telixpharma.com/news-views/prostact-global-phase-3-update-first-patient-dosed-in-randomized-treatment-expansion-part-1-readout-plans-confirmed/?utm_source=chatgpt.com










暂无评论内容