This page summarizes the latest approvals issued by the U.S. Food and Drug Administration (FDA). The list includes newly approved therapies as well as additional indications for existing agents across nephrology, oncology, rare diseases, ophthalmology, endocrinology, and other key therapeutic areas. The information is intended for healthcare professionals, institutional partners, and patients seeking timely updates on regulatory developments.
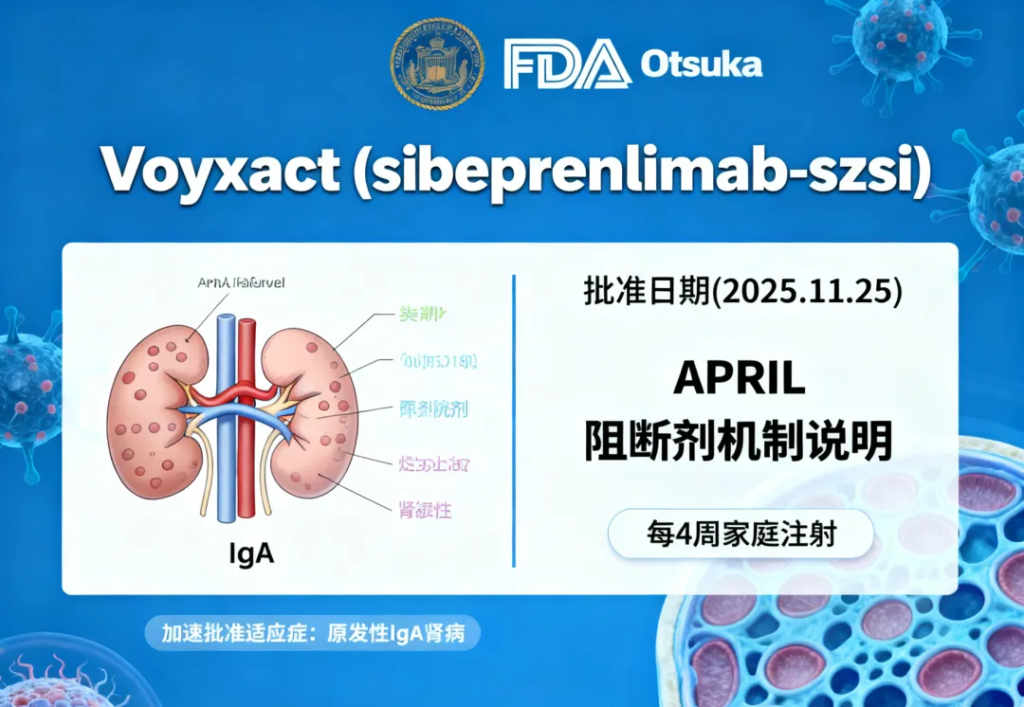
Voyxact (sibeprenlimab-szsi)
Indication: Immunoglobulin A nephropathy (IgAN)
Approval Date: November 25, 2025
An APRIL-pathway inhibitor granted accelerated approval for reducing proteinuria in adults at risk of disease progression.
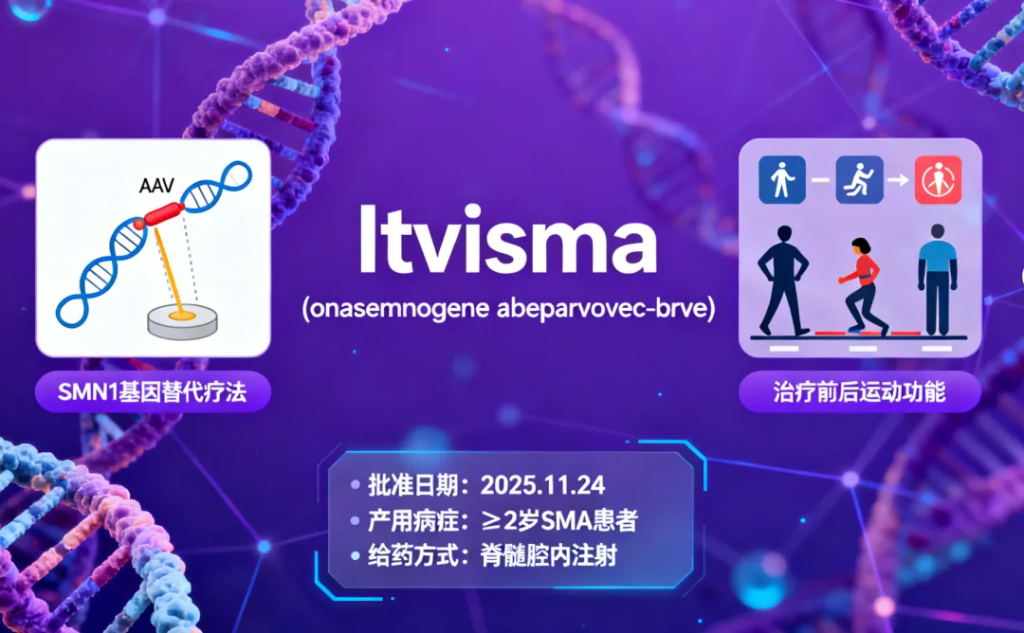
Itvisma (onasemnogene abeparvovec-brve)
Indication: Spinal muscular atrophy (SMA)
Approval Date: November 24, 2025
An adeno-associated viral (AAV) vector gene-replacement therapy for intrathecal administration in SMA patients aged 2 years and older.
Osvyrti (denosumab-desu)
Indication: Osteoporosis
Approval Date: November 20, 2025
A biosimilar to Prolia for the treatment of osteoporosis.
Jubereq (denosumab-desu)
Indications: Bone metastases, multiple myeloma–related bone disease, giant cell tumor of bone, hypercalcemia of malignancy
Approval Date: November 20, 2025
A biosimilar to Xgeva, approved for the prevention and management of skeletal-related events and tumor-associated bone conditions.
Hyrnuo (sevabertinib)
Indication: HER2-mutated non-small cell lung cancer (NSCLC)
Approval Date: November 19, 2025
A reversible tyrosine kinase inhibitor granted accelerated approval for non-squamous NSCLC harboring HER2 mutations.
Redemplo (plozasiran)
Indication: Familial chylomicronemia syndrome (FCS)
Approval Date: November 18, 2025
An siRNA therapy targeting ApoC-III, approved as an adjunct to dietary management to reduce triglyceride levels in adults with FCS.
Komzifti (ziftomenib)
Indication: Relapsed or refractory NPM1-mutated acute myeloid leukemia (AML)
Approval Date: November 13, 2025
A first-in-class menin inhibitor developed for the treatment of NPM1-mutated AML.
Poherdy (pertuzumab-dpzb)
Indication: HER2-positive breast cancer
Approval Date: November 13, 2025
An interchangeable biosimilar to Perjeta for HER2-positive breast cancer treatment.
Kygevvi (doxecitine + doxribtimine)
Indication: Thymidine kinase 2 deficiency (TK2D)
Approval Date: November 3, 2025
A combination of pyrimidine nucleosides for long-term treatment of TK2 deficiency in adults and children.
Lynkuet (elinzanetant)
Indication: Moderate to severe vasomotor symptoms associated with menopause
Approval Date: October 24, 2025
An NK1/NK3 receptor antagonist for the management of menopausal vasomotor symptoms.
Javadin (clonidine hydrochloride)
Indication: Hypertension
Approval Date: October 23, 2025
An oral solution formulation of the established α-2 adrenergic agonist.
Contepo (fosfomycin)
Indication: Complicated urinary tract infections (cUTIs)
Approval Date: October 22, 2025
An intravenous formulation of fosfomycin for treatment of cUTIs.
Epioxa (riboflavin-5’-phosphate)
Indication: Keratoconus
Approval Date: October 17, 2025
A next-generation corneal cross-linking therapy for keratoconus.
Ferabright (ferumoxytol)
Indication: MRI of intracranial malignancies
Approval Date: October 16, 2025
An iron-based contrast agent used to detect lesions associated with disruption of the blood–brain barrier.
Jascayd (nerandomilast)
Indication: Idiopathic pulmonary fibrosis (IPF)
Approval Date: October 7, 2025
A phosphodiesterase-4 inhibitor for the treatment of adult IPF.
Lasix ONYU (furosemide)
Indication: Edema associated with chronic heart failure
Approval Date: October 7, 2025
A combination product consisting of furosemide and a dedicated administration device.
Eydenzelt (aflibercept-boav)
Indications: Wet age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema, diabetic retinopathy
Approval Date: October 2, 2025
A biosimilar to Eylea for a spectrum of retinal vascular diseases.
Eltromin 25/50 Eltrombopag
Enatinib 4/10 Lenvatinib
Lynparib Olaparib
Coltinib Upadacitinib
Alvonib Osimertinib
Techno
Well-known pharmaceutical company in Bangladesh:https://www.radiantpharmacil.com
Article source:https://www.drugs.com/newdrugs.html?utm_source=chatgpt.com










暂无评论内容